Second Week of Development (Gastrulation)
- teachanatomy

- Jul 4, 2025
- 11 min read
The process of implantations begins in the first week on the 5th or 6th week and finishes in the second on the 11th or 12th day. The polar trophoblastic cells overlying the inner cell mass come in contact with the uterine epithelium and produce enzymes that erode the epithelial lining. In the second week of embryonic development, the process of implantation progresses and several crucial events that lay the foundation for the embryonic development take place. The blastocyst continues to burrow itself deep into the uterine endometrial lamina propria (endometrial stroma). The gap in the uterine epithelium caused by hydrolytic enzymes secreted by cells of the outer layer of the blastocyst (the trophoblast), gradually heals and closes off. During this time of implantation of the blastocyst, the uterine endometrium is in the secretory phase - influenced by progesterone. The lamina propria is highly cellular and contains numerous tortuous secretory uterine glands and many spiral arteries, ready to provide nutrients to the implanted developing embryo. The trophoblast - the outer layer of the blastocyst - differentiates into two layers, an inner layer called cytotrophoblast and an outer layer known as syncytiotrophoblast. Both of these two layers play a critical role in establishing the placenta that throughout pregnancy facilitate the exchange of nutrient, gases and waste products between the maternal blood and the fetal circulations. Concurrently, the inner cell mass differentiates into two distinct layers called the epiblast and the hypoblast, thus forming the bilaminar germ disk.

The epiblast will eventually give rise to the entire embryo, whereas the hypoblast contributes to the formation of the yolk sac, which provides nourishment during the early stages. Additionally, the amniotic cavity starts to form, which will later envelop the developing embryo in a protective fluid-filled sac.
During this process of implantation, the syncytiotrophoblast invades the endometrial lamina propria in such a way that maternal dilated sinusoidal capillaries invade the syncytiotrophoblast and become confluent with spaces present within the syncytiotrophoblast, known as lacunae. While every step in the process of implanting is a requisite for adequate fetal development, yet one of the most important features of the second week of embryonic development are completion of implantation and the establishment of the maternofetal vascular connections. Concomitant with the progress of the process of implantation are development changes that take place in the inner cell mass. The inner cell mass is also known as the embryoblast because it give rise to all tissues and organs of the body of the fetus. The embryoblast undergoes differentiation to form a bilaminar flat circular disc that develop into two layers, a thick epiblast made of single layer of high columnar cells and a thinner hypoblast made of a single layer of smaller cuboidal cells. A small space develops above the epiblast foreshadowing formation of the amniotic cavity.

The second week of development is thus characterized by the formation of essential structures that support and protect the embryo, setting the stage for the intricate processes that will follow in the subsequent weeks; these include the epiblast, the hypoblast, the yolk sac, and the amniotic cavity.
The Epiblast
The inner cell mass – also known as the embryoblast - develops at the time transformation of the morula into a blastocyst, as cluster of cells present within the blastocyst overlying the newly formed cavity, the blastocoel. Later on during implantation of the blastocyst, morphologic changes in the in embryoblast produce a bilaminar embryonic disc consisting of two distinct layers - an epiblast and a hypoblast; the epiblast overlies the hypoblast. The can be clearly seen in blastocysts of the second week of embryonic development as layer of columnar cells.

The epiblast is also known as the primary ectoderm; it gives rise to the three germ layers namely the definitive ectoderm, mesoderm and endoderm. Cells of the epiblast are pluripotent cells capable of differentiating into a wide array of functional cells including nerve cells, the epidermal cells of the skin and sensory cells of the eye and ear. Moreover, it contributes to the formation of the extraembryonic membranes. It is responsible for the formation of the primitive streak, a structure that plays a pivotal role in the process of gastrulation. Epiblast is the source of the germ cells that migrate and invaginate in the primitive streak to differentiate into all cells of the fetus, including the male and female germ cells.
The Hypoblast
The hypoblast is the deeper or inner layer of the embryoblast (the embryonic disc). It is made of cuboidal cells and contributes to the formation of the primitive endoderm and the yolk sac.

The Primitive Yolk Sac
The primitive yolk sac, also known as the primary umbilical vesicle, or the primary yolk sac, is a membranous sac present outside the embryonic dis. The primary yolk sac and the amniotic appear in about the time during implantation of the blastocyst on the 8th day of embryonic development. The primitive yolk sac develops by proliferation of cells of the hypoblast after implantation of the blastocyst and occupy the cavity of the blastocyst replacing the blastocoel. Later on in the subsequent weeks, the primary yolk sac enlarges, gains a lining of the extraembryonic mesoderm, loses part of itself and transforms into the secondary yolk sac. The yolk sac gives rise to the male and female primordial germ cells that ultimately differentiate into spermatogonia and oogonia. It also participates in the formation of the chorion and the allantois.

The amnion
A tiny space called the amniotic cavity forms between the epiblast and cytotrophoblast in the beginning of the second week of the embryonic development. Cells migrate upwards from the epiblast to form a thin membrane called the amnion; this membrane surrounds the amniotic cavity and separates it from the cytotrophoblast. The amniotic cavity fills up with a fluid called the amniotic fluid. The amnion is a saclike structure that directly surrounds the fetus; it protects the developing embryo and fetus from pressure serving as cushion or a shock absorber and preventing adhesions at the same time. Towards the end of the second week of embryonic development and after formation of the amnion, cells migrate from the trophoblast and the extraembryonic somatic mesoderm form a membrane that encloses a cavity called the chorionic cavity.

Twinning
Twinning is giving birth to more than one baby at the end of a gestation. A twin refers to two or more children born together at same birth but often refers to two children born; three born together are called a triplet. There are two types of twins, identical or monozygotic and fraternal or dizygotic or polyzygotic twins. Identical twins develop from a single fertilized ovum (one zygote) that splits at one or another stage of its development, whereas fraternal twins develop from two or more separate zygotes. In case of fraternal twins two or more oocytes are ovulated as the same time, fertilized by different sperms and each fertilized ovum (zygote) develops and yields an offspring. These offsprings are no more alike than siblings born at separate times; they could be a boy and a girl, two girls or two boys; i.e. they are of the same sex or opposite sexes and their genetic make ups are as different as other siblings who are not twins. They have different hair or eye color or complexion.
Identical twins share the same genome; they have the same genome as that of the zygote they developed from. They will be of the same sex and the same features. There are different types of identical twins which include dichorionic twins, monochorionic diamniotic and monochorionic monoamniotic twins. These differences arise from the difference in the time where splitting of the developing embryo takes place.
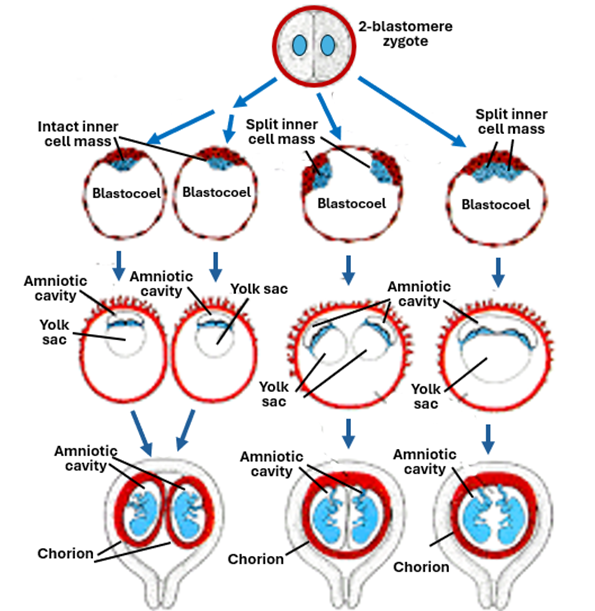
Dichorionic diamniotic monozygotic twins arise in the early stages of embryonic development, from the beginning cleavage until the morula stage. The incidence of monozygotic twinning is about 0.04%. During this period a strong zona pellucida surrounds the blastomeres and prevents their separation from each especially that the blastomeres are not held together firmly by intercellular junctions. Weakness of the zona pellucida allows the blastomeres to split apart into two groups of cell each regaining its own zona pellucida. Each of the two groups continues development into the blastocyst stages. Each of the two blastocysts implants itself into the uterine endometrium and develops independently of the other forming its own amnion, chorion, other fetal membranes and the placenta. In this respect they resemble nonidentical fraternal twins.

Monochorionic diamniotic monozygotic twins develop in the blastocyst stage. The inner cell mass splits into two groups of cells before formation of the amnion. Each group will develop its own amniotic sac but will share the chorionic sac with the other twin embryo. The two embryos share the same placenta and one chorionic sac, but each embryo will have its own amniotic sac that separates it from the other twin.

Monochorionic Monoamniotic monozygotic twins also develop in the blastocyst stage, but the inner cell mass splits into two groups of cells after formation of the amnion. Thus, both developing embryos share the same amniotic sac and the same chorionic sac and the placenta. The two developing embryos may come in contact with each leading to adhesions and possibly formation of conjoined twins.

Thus, early splitting of the embryo will lead to formation of twins, each possessing its own set fetal membranes, whereas splitting of the embryo at later stages leads to formation of twins sharing fetal membranes. Splitting before formation of the amnion leads to formation of twins sharing the same chorion but each with its own amnion. On the other hand, splitting of the developing embryo (inner cell mass) after formation of the amnion leads to formation of twins sharing both the amnion and the chorion.
Conjoined twins
Conjoined twins are twins that get joined to each other during embryonic development; they are commonly referred to as Siamese twins. It is a rare condition with an incidence rat of about 1:100,000. More than 60% of them are either stillborn or die within 24 hours of birth. They occur in monozygotic monoamniotic monochorionic twins. It may occur due to partial splitting of the inner cells or fusion of the two embryo during development that follows complete splitting of the inner cell mass. Conjoined twins are classified according to the location and degree of the union between the two of them. According to the location of the union, conjoined twins are classified into:
Thoracopagus is the condition when the twins are joined together in the thoracic region of the body. It is the most common type of conjoined twins constituting about one third of the cases (30%). Twins of this kind often share the heart and the liver and have a poor survival rate. The heart is shared between them and the survival rate is also poor.
Thoraco-omphalopagus is a condition where the twins a conjoined in the thoracic region and abdominal regions, and its incidence rate is about 20%. They share the heart, and the survival rate is poor but can improve of one of the twins is sacrificed.
Omphalopagus is a condition where identical twins are joined together in lower part of the abdomen. The conjoined twins share the liver and parts of the alimentary tract but not the heart. Survival rate is good, reaching about 80%.
Craniopagus is a condition where the skulls of the twins are fused together. It has an incidence rate of about 5%, and the survival rate is poor.
Heteropagus is a congenital anomaly where the twins of different size, one small and the other large. The smaller one is less developed than the larger one and depends on its survival on the larger one and accordingly called the parasitic twin.

Contraception
Contraception is prevention of conception or pregnancy; it is a renowned method for birth control. It aims at averting sperms from reaching the site of fertilization and hindering implantation. It is classified into four main categories, namely hormonal contraception, barrier contraception, intrauterine device (IUD) contraception and permanent contraception.
Hormonal contraception is accomplished mainly by female sex hormones usually by a combination of estrogen and progesterone or progesterone alone. It is used in the form of oral pills or skin patches to prevent ovulation. It inhibits ovulation by suppressing follicular development. Progestogen induces negative feedback in the hypothalamus that decrease production of gonadotropin-releasing hormone. According, no secondary oocytes are released by the ovary to be available for sperms to fertilize and produce a zygote.
Barrier contraception aims at creating a barrier that prevents access of sperms to the site of fertilization. It includes the use of condoms, cervical diaphragms, cervical caps, contraceptive vaginal sponges and vaginal spermicides. They all impose a physical barrier against entrance of sperms into the uterus preventing them gaining access to oocytes in the fallopian tube, thus preventing union of the male and female gametes.
IUD contraception depends on insertion of devices into the uterine lumen (intrauterine devices). It is a long-acting method of contraception. Intrauterine devices contain progesterone or copper. Copper induces an inflammatory reaction in the endometrium and makes the uterine chamber hostile to sperms and ova, whereas progesterone works by the same mechanism described above.
Permanent contraception utilizes surgical intervention to block the way for sperms and ova to meet each other. It includes vasectomy and tube ligation. Vasectomy aims at sealing the vas deferens to hinder ejaculation of sperms from the epididymis outwards. Thus, after vasectomy no sperms are deposited into the female vagina following an intercourse. Tube ligation is tying up the Fallopian tube to prevent transport of the oocyte down the tube. Thus, there will be no chance of sperms to reach the oocyte and fertilize it.




Comments